Cream of Tartar What Is It Made From
"Cream of tartar" redirects here. For the sauce, see Tartar sauce.
 | |||
| |||
| Names | |||
|---|---|---|---|
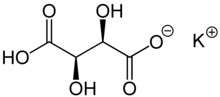
| Preferred IUPAC name Potassium (2R,3R)-2,3,4-trihydroxy-4-oxobutanoate | |||
| Other names Potassium hydrogen tartrate | |||
| Identifiers | |||
| CAS Number |
| ||
| 3D model (JSmol) |
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.011.609 | ||
| E number | E336 (antioxidants, ...) | ||
| PubChem CID |
| ||
| UNII |
| ||
| InChI
| |||
| SMILES
| |||
| Properties | |||
| Chemical formula | KC4H5O6 | ||
| Molar mass | 188.177 | ||
| Appearance | white crystalline powder | ||
| Density | 1.05 g/cm3 (solid) | ||
| Solubility in water | 0.57 g/100ml (20 °C) 6.1 g/100ml (100 °C) | ||
| Solubility | soluble in acid, alkali insoluble in acetic acid, alcohol | ||
| Refractive index (n D) | 1.511 | ||
| Pharmacology | |||
| ATC code | A12BA03 (WHO) | ||
| Hazards | |||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 22 g/kg (oral, rat) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Chemical compound
Potassium bitartrate, also known as potassium hydrogen tartrate, with formula KC4H5O6, is a byproduct of winemaking. In cooking, it is known as cream of tartar. It is processed from the potassium acid salt of tartaric acid (a carboxylic acid). The resulting powdery base can be used in baking or as a cleaning solution (when mixed with an acidic solution such as lemon juice or white vinegar).
Occurrence [edit]

Potassium bitartrate in an empty white wine bottle
Potassium bitartrate has a low solubility in water. It crystallizes in wine casks during the fermentation of grape juice, and can precipitate out of wine in bottles. The crystals (wine diamonds) will often form on the underside of a cork in wine-filled bottles that have been stored at temperatures below 10 °C (50 °F), and will seldom, if ever, dissolve naturally into the wine.
These crystals also precipitate out of fresh grape juice that has been chilled or allowed to stand for some time.[1] To prevent crystals forming in homemade grape jam or jelly, the prerequisite fresh grape juice should be chilled overnight to promote crystallization. The potassium bitartrate crystals are removed by filtering through two layers of cheesecloth. The filtered juice may then be made into jam or jelly.[2] In some cases they adhere to the side of the chilled container, making filtering unnecessary.
The crude form (known as beeswing) is collected and purified to produce the white, odorless, acidic powder used for many culinary and other household purposes.
Applications [edit]
In food [edit]

Folger's Golden Gate Cream Tartar, first half of 20th century
In food, potassium bitartrate is used for:
- Stabilizing egg whites, increasing their warmth tolerance and volume[3]
- Stabilizing whipped cream, maintaining its texture and volume[4]
- Anti-caking and thickening[5]
- Preventing sugar syrups from crystallizing, by causing some of the sucrose to break down into glucose and fructose[6]
- Reducing discoloration of boiled vegetables
Additionally it is used as a component of:
- Baking powder, as an acid ingredient to activate baking soda[7]
- Sodium-free salt substitutes, in combination with potassium chloride
A similar acid salt, sodium acid pyrophosphate, can be confused with cream of tartar because of its common function as a component of baking powder.
Household use [edit]
Potassium bitartrate can be mixed with an acidic liquid such as lemon juice or white vinegar to make a paste-like cleaning agent for metals such as brass, aluminium or copper, or with water for other cleaning applications such as removing light stains from porcelain.[8] This mixture is sometimes mistakenly made with vinegar and sodium bicarbonate (baking soda), which actually react to neutralize each other, creating carbon dioxide and a sodium acetate solution.
Cream of tartar was often used in traditional dyeing where the complexing action of the tartrate ions was used to adjust the solubility and hydrolysis of mordant salts such as tin chloride and alum.
Cream of tartar, when mixed into a paste with hydrogen peroxide, can be used to clean rust from some hand tools, notably hand files. The paste is applied and allowed to set for a few hours and then washed off with a baking soda/water solution. After another rinse with water and thorough drying, a thin application of oil will protect the file from further rusting.
Slowing the set time of plaster of Paris products (most widely used in gypsum plaster wall work and artwork casting) is typically achieved by the simple introduction of almost any acid diluted into the mixing water. A commercial retardant premix additive sold by USG to trade interior plasterers includes at least 40% potassium bitartrate. The remaining ingredients are the same plaster of Paris and quartz-silica aggregate already prominent in the main product. This means that the only active ingredient is the cream of tartar.[9]
Cosmetics [edit]
For dyeing hair, potassium bitartrate can be mixed with henna as the mild acid needed to activate the henna.
Medicinal use [edit]
Cream of tartar has been used internally as a purgative, but this is dangerous because an excess of potassium, or hyperkalemia, may occur.[10] [ citation needed ]
Chemistry [edit]
Potassium bitartrate is the United States' National Institute of Standards and Technology's primary reference standard for a pH buffer. Using an excess of the salt in water, a saturated solution is created with a pH of 3.557 at 25 °C (77 °F). Upon dissolution in water, potassium bitartrate will dissociate into acid tartrate, tartrate, and potassium ions. Thus, a saturated solution creates a buffer with standard pH. Before use as a standard, it is recommended that the solution be filtered or decanted between 22 °C (72 °F) and 28 °C (82 °F).[11]
Potassium carbonate can be made by burning cream of tartar, which produces "pearl ash". This process is now obsolete but produced a higher quality (reasonable purity) than "potash" extracted from wood or other plant ashes.
Production [edit]
| | This section needs expansion. You can help by adding to it. (August 2019) |
See also [edit]
- Tartrate
- Tartaric acid
- Potassium tartrate (K2C4H4O6)
References [edit]
- ^ Max Williams at McNicol Williams Management & Marketing Services. "Lloyds Vinyard FAQs". Lloydsvineyard.com.au. Archived from the original on 15 December 2011. Retrieved 19 April 2018.
- ^ "National Center for Home Food Preservation". Uga.edu. Retrieved 19 April 2018.
- ^ The science of good cooking : master 50 simple concepts to enjoy a lifetime of success in the kitchen (1st ed.). America's Test Kitchen. 2012. p. 199. ISBN978-1-933615-98-1.
- ^ "How to Use Cream of Tartar". wikiHow . Retrieved 28 May 2019.
- ^ Stephens, Emily (18 February 2017). "The Incredible Cream of Tartar – How to Use and What to Substitute With". MyGreatRecipes . Retrieved 28 May 2019.
- ^ Provost, Joseph J.; Colabroy, Keri L.; Kelly, Brenda S.; Wallert, Mark A. (2016). The science of cooking : understanding the biology and chemistry behind food and cooking. John Wiley and Sons, Inc. p. 504. ISBN9781118674208.
- ^ McGee, Harold (2004). On food and cooking : the science and lore of the kitchen (2nd ed.). Scribner. p. 533,534. ISBN978-0-684-80001-1.
- ^ "Michigan State University Extension Home Maintenance And Repair – Homemade Cleaners – 01500631, 06/24/03". Archived from the original on 23 June 2009. Retrieved 19 April 2018.
- ^ "Material Safety Data Sheet: Gypsum Plaster Retarder for Lime-Based Products" (PDF). USG Inc. Archived from the original (PDF) on 29 August 2016. Retrieved 21 July 2016.
- ^ Rusyniak, Daniel E.; Durant, Pamela J.; Mowry, James B.; Johnson, Jo A.; Sanftleben, Jayne A.; Smith, Joanne M. (2013). "Life-Threatening Hyperkalemia from Cream of Tartar Ingestion". Journal of Medical Toxicology. 9 (1): 79–81. doi:10.1007/s13181-012-0255-x. PMC3570668. PMID 22926733.
- ^ Harris, Daniel C. (17 July 2006), Quantitative Chemical Analysis (7th ed.), New York: W. H. Freeman, ISBN978-0-7167-7694-9
External links [edit]
- Description of Potassium Bitartrate at Monash Scientific
- Material Safety Data Sheet (MSDS) for Potassium Bitartrate at Fisher Scientific
-
 This article incorporates text from a publication now in the public domain:Ward, Artemas (1911). The Grocer's Encyclopedia.
This article incorporates text from a publication now in the public domain:Ward, Artemas (1911). The Grocer's Encyclopedia.
Cream of Tartar What Is It Made From
Source: https://en.wikipedia.org/wiki/Potassium_bitartrate


0 Response to "Cream of Tartar What Is It Made From"
Post a Comment